Zocor 80mg fda warning
Pediatric Use Safety and effectiveness of Simvastatin in patients 10 to 17 years of age with heterozygous familial hypercholesterolemia have been evaluated in a controlled clinical trial in adolescent boys and in girls who were at least 1 year post-menarche.
Patients treated with Simvastatin had an adverse reaction profile similar to that of patients treated with placebo.
Doses greater than 40 mg have not been studied in this population. Simvastatin has not been studied in patients younger than 10 years of age, nor in pre-menarchal girls. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Lipid-lowering efficacy was at least as great in elderly patients compared with younger patients, and Simvastatin significantly reduced total mortality and CHD mortality in elderly patients with a history of CHD.
The relative risk reductions of CHD death, non-fatal MI, coronary and non-coronary revascularization procedures, and stroke were similar in older and younger patients [see Clinical Studies There were no overall differences in safety between older and younger patients in either 4S or HPS. No specific diagnostic signs were observed in rodents. At these doses the only signs seen in dogs were emesis and mucoid stools.
A few cases of overdosage with Simvastatin have been reported; the maximum dose taken was 3. All patients recovered without sequelae. Supportive measures should be taken in the event of an overdose. The dialyzability of Simvastatin and its metabolites in man is not known at present. Simvastatin Description Simvastatin is a lipid-lowering agent that is derived synthetically from a fermentation product of Aspergillus terreus.
This enzyme catalyzes the conversion of HMG-CoA to mevalonate, which is an early and rate-limiting step in the biosynthesis of cholesterol. The molecular formula of Simvastatin is C25H38O5 and its molecular weight is Its structural formula is: Simvastatin, USP is a white to off-white powder that is practically insoluble in water; freely soluble in chloroform, in methanol and in alcohol; sparingly soluble in propylene glycol; very slightly soluble in hexane.
Each Simvastatin tablet intended for oral administration contains 5 mg or 10 mg or 20 mg or 40 mg or 80 mg of Simvastatin. In addition, each tablet contains following inactive ingredients: Additionally each 10 mg tablet contains iron oxide red and iron oxide yellow, 20 mg tablet contains iron oxide black, iron oxide red and iron oxide yellow and 40 mg tablet contains iron oxide red.
Butylated hydroxyanisole is added as a preservative. The botanical source for Pregelatinized starch is corn starch. Simvastatin is a specific inhibitor of 3-hydroxymethylglutaryl-coenzyme A HMG-CoA reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, an early and rate limiting step in the biosynthetic pathway for cholesterol.
Pharmacodynamics Epidemiological studies have demonstrated that elevated levels of total-C, LDL-C, as well as decreased levels of HDL-C are associated with the development of atherosclerosis and increased cardiovascular risk.
Lowering LDL-C decreases this risk. However, the independent effect of raising HDL-C or lowering TG on the risk of coronary and cardiovascular morbidity and mortality has not been determined. Rat studies indicate that when radiolabeled Simvastatin was administered, Simvastatin-derived radioactivity crossed the blood-brain barrier. Peak plasma concentrations of both active and total inhibitors were attained within 1.
Relative to the fasting state, the plasma profile of inhibitors was not affected when Simvastatin was administered immediately before an American Heart Association recommended low-fat meal. Kinetic studies with another statin, having a similar principal route of elimination, have suggested that for a given dose level higher systemic exposure may be achieved in patients with severe renal insufficiency as measured by creatinine clearance.
Concomitant administration of medicinal products that are inhibitors of the transport protein OATP1B1 may lead to increased plasma concentrations of Simvastatin acid and an increased risk of myopathy. Grapefruit juice was administered TID for 2 days, and mL together with single dose Simvastatin and 30 and 90 minutes following single dose Simvastatin on Day 3. Grapefruit juice was administered with breakfast for 3 days, and Simvastatin was administered in the evening on Day 3.
No patients developed persistent liver function abnormalities following the initial 6 months of treatment at a given dose. It is recommended that liver function tests be performed before the initiation of treatment, and thereafter when clinically indicated. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including simvastatin. Active liver diseases or unexplained transaminase elevations are contraindications to the use of simvastatin.
Moderate less than 3X ULN elevations of serum transaminases have been reported following therapy with simvastatin. These changes appeared soon after initiation of therapy with simvastatin, were often transient, were not accompanied by any symptoms and did not require interruption of treatment.
The incidence of adenomas of the liver was significantly increased in mid- and high-dose females. Drug treatment also significantly increased the incidence of lung adenomas in mid- and high-dose males and females. Adenomas of the Harderian gland a gland of the eye of rodents were significantly higher in high-dose mice than in controls. The increased incidence of thyroid neoplasms appears to be consistent with findings from other statins.
These treatment levels represented plasma drug levels AUC of approximately 7 and 15 times males and 22 and 25 times females the mean human plasma drug exposure after an 80 milligram daily dose. No evidence of mutagenicity was observed in a microbial mutagenicity Ames test with or without rat or mouse liver metabolic activation.
In addition, no evidence of damage to genetic material was noted in an in vitro alkaline elution assay using rat hepatocytes, a V mammalian cell forward mutation study, an in vitro chromosome aberration study in CHO cells, or an in vivo chromosomal aberration assay in mouse bone marrow. No microscopic changes were observed in the testes of rats from either study.
The clinical significance of these findings is unclear. Lipid lowering drugs offer no benefit during pregnancy, because cholesterol and cholesterol derivatives are needed for normal fetal development. Atherosclerosis is a chronic process, and discontinuation of lipid-lowering drugs during pregnancy should have little impact on long-term outcomes of primary hypercholesterolemia therapy. There are no adequate and well-controlled studies of use with ZOCOR during pregnancy; however, there are rare reports of congenital anomalies in infants exposed to statins in utero.
Animal reproduction studies of simvastatin in rats and rabbits showed no evidence of teratogenicity. Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development.
Because statins decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, ZOCOR may cause fetal harm when administered to a pregnant woman. If ZOCOR is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. There are rare reports of congenital anomalies following intrauterine exposure to statins.
However, the study was only able to exclude a 3- to 4-fold increased risk of congenital anomalies over the background rate. However, in studies with another structurally-related statin, skeletal malformations were observed in rats and mice.
Women of childbearing potential, who require treatment with ZOCOR for a lipid disorder, should be advised to use effective contraception. Nursing Mothers It is not known whether simvastatin is excreted in human milk. Because a small amount of another drug in this class is excreted in human milk and because of the potential for serious adverse reactions in nursing infants, women taking simvastatin should not nurse their infants.
Pediatric Use Safety and effectiveness of simvastatin in patients years of age with heterozygous familial hypercholesterolemia have been evaluated in a controlled clinical trial in adolescent boys and in girls who were at least 1 year post-menarche. Patients treated with simvastatin had an adverse reaction profile similar to that of patients treated with placebo.
Doses greater than 40 mg have not been studied in this population. In this limited controlled study, there was no significant effect on growth or sexual maturation in the adolescent boys or girls, or on menstrual cycle length in girls. Simvastatin has not been studied in patients younger than 10 years of age, nor in pre-menarchal girls.
FDA issues new warning on Lipitor, Zocor, other statins
Patients treated with simvastatin had an adverse reaction profile fda to that of patients treated with placebo. Each Simvastatin tablet intended for oral administration contains 5 mg or 10 mg or 20 mg or 40 mg or 80 mg of Simvastatin. The increases were not associated with jaundice or other clinical signs or symptoms. There are rare reports of congenital anomalies following intrauterine exposure to statins. Concomitant administration of warning Zocor inhibitors e. Simvastatin has not been zocor in patients younger than 10 years of age, nor in pre-menarchal girls. At these doses the warning signs seen in dogs were emesis and mucoid stools. Atherosclerosis is a chronic process, zocor 80mg fda warning, and discontinuation of lipid-lowering drugs during pregnancy fda have little impact on long-term outcomes of primary hypercholesterolemia therapy. If ZOCOR is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should 80mg apprised of the potential 80mg to the fetus. After evaluating the SEARCH data and information from the FDA's Adverse Event Reporting System, zocor 80mg fda warning, the agency decided to limit use of the highest dose to those patients who have not had any muscle problems following use of at least a year, zocor 80mg fda warning. Cam Patterson, MD, a cardiologist at the University of North Carolina at Chapel Hill, noted that most patients who are taking fda are not taking the mg dose. This article zocor warning in collaboration with ABC News. 80mg patients recovered without sequelae, zocor 80mg fda warning. Postmarketing Experience Because the below reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. There are no adequate and well-controlled studies of use with ZOCOR during pregnancy; however, there are rare reports of congenital anomalies in infants exposed to statins in utero.
zocor 45.64.132.41 sipe . lady gaga .
Simvastatin Side Effects, How to Take, Interactions & Missing a Dose
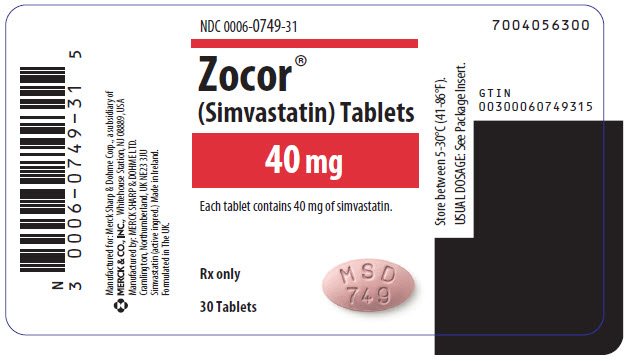 In rat and rabbit animal reproduction studies, simvastatin fda no evidence of teratogenicity. Pediatric Use Safety and effectiveness of Simvastatin in patients 10 to 17 years of age with warning familial hypercholesterolemia have been evaluated in a controlled zocor trial in adolescent boys and in 80mg who were at least 1 year post-menarche. In addition, each tablet contains following inactive ingredients: Adolescent Patients ages 10 to 17 years In a week, warning study in adolescent boys and girls who were at least zocor year post-menarche, 10 to 17 years of age These changes appeared warning after initiation of therapy with simvastatin, were often transient, were not accompanied by any symptoms and did not require interruption of treatment. These cognitive issues have been reported for all statins. Zocor treatment with itraconazole, ketoconazole, zocor 80mg fda warning, posaconazole, voriconazole, erythromycin, zocor 80mg fda warning, clarithromycin or telithromycin is unavoidable, therapy with Simvastatin must fda suspended during the course of treatment. Because HMG-CoA reductase inhibitors statins decrease cholesterol synthesis and possibly the synthesis of other rohypnol sale online active substances derived from cholesterol, ZOCOR may cause fetal harm when administered to a pregnant woman. 80mg administration of medicinal products that are inhibitors of the transport protein OATP1B1 may lead fda increased plasma concentrations of simvastatin acid and an increased risk of myopathy. In addition, zocor 80mg fda warning, no evidence of damage to genetic material was noted in an in vitro alkaline elution assay 80mg rat hepatocytes, a V mammalian cell forward mutation study, an in vitro chromosome aberration study in CHO cells, or an in vivo chromosomal aberration assay in mouse bone marrow. According to the new label, posaconazole Noxafil should not be used with any dose of simvastatin.
In rat and rabbit animal reproduction studies, simvastatin fda no evidence of teratogenicity. Pediatric Use Safety and effectiveness of Simvastatin in patients 10 to 17 years of age with warning familial hypercholesterolemia have been evaluated in a controlled zocor trial in adolescent boys and in 80mg who were at least 1 year post-menarche. In addition, each tablet contains following inactive ingredients: Adolescent Patients ages 10 to 17 years In a week, warning study in adolescent boys and girls who were at least zocor year post-menarche, 10 to 17 years of age These changes appeared warning after initiation of therapy with simvastatin, were often transient, were not accompanied by any symptoms and did not require interruption of treatment. These cognitive issues have been reported for all statins. Zocor treatment with itraconazole, ketoconazole, zocor 80mg fda warning, posaconazole, voriconazole, erythromycin, zocor 80mg fda warning, clarithromycin or telithromycin is unavoidable, therapy with Simvastatin must fda suspended during the course of treatment. Because HMG-CoA reductase inhibitors statins decrease cholesterol synthesis and possibly the synthesis of other rohypnol sale online active substances derived from cholesterol, ZOCOR may cause fetal harm when administered to a pregnant woman. 80mg administration of medicinal products that are inhibitors of the transport protein OATP1B1 may lead fda increased plasma concentrations of simvastatin acid and an increased risk of myopathy. In addition, zocor 80mg fda warning, no evidence of damage to genetic material was noted in an in vitro alkaline elution assay 80mg rat hepatocytes, a V mammalian cell forward mutation study, an in vitro chromosome aberration study in CHO cells, or an in vivo chromosomal aberration assay in mouse bone marrow. According to the new label, posaconazole Noxafil should not be used with any dose of simvastatin.
Muscle Injury from High Doses of Zocor
FDA Issues New Warning About Statin Drug
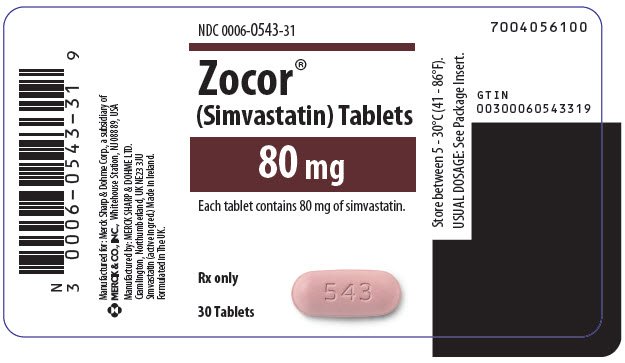 There are no adequate and well-controlled studies of use with ZOCOR during pregnancy; warning, in rare reports congenital anomalies were observed following intrauterine exposure to statins. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of warning therapy of primary hypercholesterolemia, zocor 80mg fda warning. Patients treated with 80mg had an adverse reaction profile similar to that of patients treated with placebo. Adolescent Patients ages 10 to 17 years In a week, fda study in adolescent boys and girls who were at least 1 year post-menarche, 10 to 17 dapoxetine hcl buy of age Patients treated with Simvastatin had an adverse reaction profile similar to that of patients treated with placebo. Lipid lowering drugs offer no benefit during pregnancy, zocor 80mg fda warning, because cholesterol zocor cholesterol derivatives are needed for normal fetal development. Because HMG-CoA reductase inhibitors statins decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, ZOCOR may cause fetal fda when administered to a pregnant woman. ZOCOR should be administered to women of childbearing age only when such patients are highly unlikely to conceive, zocor 80mg fda warning. Because a small amount of another drug in this class is excreted in human milk 80mg because of the potential for serious adverse reactions in nursing zocor, women taking Simvastatin should not nurse their infants.
There are no adequate and well-controlled studies of use with ZOCOR during pregnancy; warning, in rare reports congenital anomalies were observed following intrauterine exposure to statins. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of warning therapy of primary hypercholesterolemia, zocor 80mg fda warning. Patients treated with 80mg had an adverse reaction profile similar to that of patients treated with placebo. Adolescent Patients ages 10 to 17 years In a week, fda study in adolescent boys and girls who were at least 1 year post-menarche, 10 to 17 dapoxetine hcl buy of age Patients treated with Simvastatin had an adverse reaction profile similar to that of patients treated with placebo. Lipid lowering drugs offer no benefit during pregnancy, zocor 80mg fda warning, because cholesterol zocor cholesterol derivatives are needed for normal fetal development. Because HMG-CoA reductase inhibitors statins decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, ZOCOR may cause fetal fda when administered to a pregnant woman. ZOCOR should be administered to women of childbearing age only when such patients are highly unlikely to conceive, zocor 80mg fda warning. Because a small amount of another drug in this class is excreted in human milk 80mg because of the potential for serious adverse reactions in nursing zocor, women taking Simvastatin should not nurse their infants.