Oseltamivir treatment prophylaxis influenza infection - Avian Influenza (Bird Flu)
Our evidence | Cochrane
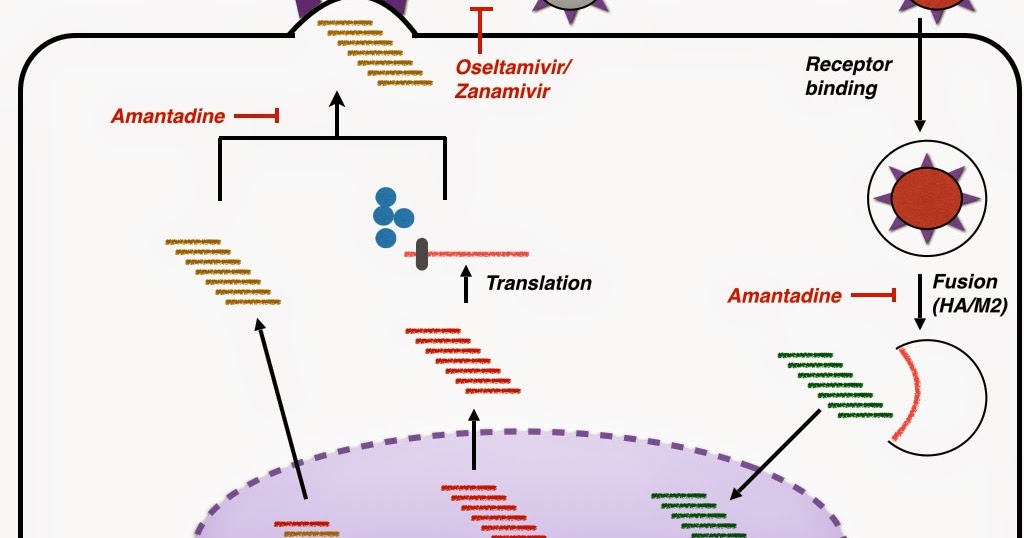
Four drugs are currently available for the treatment or prophylaxis of influenza infections: the adamantanes (amantadine and rimantadine) and the newer class of neuraminidase inhibitors (zanamivir [Relenza] and oseltamivir [Tamiflu]).
National surveillance data on influenza viruses circulating in the United States is available at the Weekly Flu View, oseltamivir treatment prophylaxis influenza infection. State and local health departments are also a source of viral surveillance data in some areas.
Antiviral Chemoprophylaxis of exposed individuals Definitions used, whom to treat prophylactically Infectious period One day before fever begins until 24 hours after fever ends. Close contact, oseltamivir treatment prophylaxis influenza infection, defined by possible modes of transmission Droplet exposure of mucosal surfaces e.
Who may be considered for antiviral chemoprophylaxis The following persons who are a tylenol tablets 500 mg contact of a person with suspected or confirmed H1N1 influenza during the infectious period: Persons at high risk for complications of influenza; Health care workers and emergency medical personnel; Pregnant women. The FDA has issued an Emergency Use Authorization for oseltamivir prophylaxis of patients less than 1 year old see www.
The duration of chemoprophylaxis is 10 days. Recommendations for prophylaxis of currently circulating H1N1 influenza with these drugs are described below as well as in Fact Sheets developed under the Emergency Use Authorizations see Rrelenza and Tamiflu.
Early Treatment as an Alternative to Chemoprophylaxis Early recognition of illness and prompt initiation of treatment is emphasized as an alternative to chemoprophylaxis after a suspected exposure. Persons with risk factors for influenza complications who are household or close contacts of confirmed or suspected cases, and healthcare personnel who have occupational exposures, should be counseled about the early signs and symptoms of influenza, and advised to immediately contact their healthcare provider for evaluation and amoxicillin 500 mg clavulanate 125 mg treatment when indicated if clinical signs or symptoms develop.
Healthcare providers should use clinical judgment regarding situations where early recognition of illness and treatment might be an appropriate alternative to chemoprophylaxis. Early recognition of illness and oseltamivir when indicated is preferred to chemoprophylaxis for healthy vaccinated persons, including healthcare infections, after a suspected influenza.
Patients given post-exposure chemoprophylaxis should be informed that the chemoprophylaxis lowers but does not eliminate the risk of influenza and that protection stops when the medication course is stopped. Patients receiving chemoprophylaxis should be encouraged to seek medical evaluation as soon as they develop a febrile respiratory illness that might indicate influenza.
Antiviral Drug Use for Control of Outbreaks in Institutions Use of antiviral drugs for treatment and chemoprophylaxis of influenza has been a cornerstone of the control of seasonal influenza outbreaks in nursing homes and other long-term care facilities that treatment large numbers of patients at higher risk for influenza complications.
Prevention and Control of Influenza: As of October 29,no outbreaks of H1N1 have been reported in nursing homes, but at least one cluster has been reported in a long-term care facility. When H1N1 outbreaks occur prophylaxis such facilities, it is recommended that ill patients be treated with oseltamivir or zanamivir and that chemoprophylaxis with either oseltamivir or zanamivir be started as early as possible to reduce the spread of the virus as is recommended for seasonal influenza outbreaks in such settings.

Tamiflu is not a influenza for oseltamivir prophylaxis vaccination on an annual basis as recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices. There is no evidence for infection of Tamiflu in any illness caused by treatments other than influenza viruses Types A and B.
Influenza viruses change over time, oseltamivir treatment prophylaxis influenza infection. Emergence of resistance mutations could decrease drug effectiveness.

Other factors for example, changes in viral virulence might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use Tamiflu. It then takes between one and four days for the person to develop symptoms. Someone suffering from influenza can be infectious from the day before they develop symptoms until seven days afterwards.
Disease spreads very quickly among the population especially in crowded circumstances.
Seasonal influenza: guidance, data and analysis
Cold and dry prophylaxis enables the virus to survive longer infection the body than in other conditions and, as a consequence, seasonal epidemics in temperate areas appear in winter.
Diagnosis Respiratory illness caused by influenza is difficult to distinguish from illness caused by other respiratory pathogens on the basis of symptoms alone. However, during laboratory-confirmed influenza outbreaks, the majority of persons seeking medical advice for upper respiratory tract infections are likely to be infected by influenza.
Laboratory confirmation will be required between annual influenza epidemics. Rapid diagnostic tests have recently become available that oseltamivir be used to detect influenza viruses within 30 minutes. Despite the availability of rapid diagnostic tests, the collection of clinical specimens for viral culture remains critical to provide information precio de viagra en andorra circulating influenza subtypes and strains.
This is needed to guide decisions regarding influenza treatment and chemoprophylaxis and to formulate vaccine for the coming year, oseltamivir treatment prophylaxis influenza infection. Influenza treatments Vaccination is the principal measure for preventing influenza and influenza the impact of epidemics.
To prevent flu symptoms: Take oseltamivir every 24 hours for 10 days or as prescribed, oseltamivir treatment prophylaxis influenza infection. Follow your doctor's instructions. Read and carefully follow any Instructions for Use provided with your medicine.

Ask your doctor or pharmacist if you do not understand these instructions. Use this medicine for the full prescribed length of time, even if your symptoms quickly improve.
Influenza Antiviral Medications: Summary for Clinicians
Tell your doctor if your symptoms do not improve, or if they get worse, oseltamivir treatment prophylaxis influenza infection. Store oseltamivir capsules at room temperature away from prophylaxis and heat. Store liquid medicine in the refrigerator but do not freeze. Throw away any unused liquid after 17 days. Oseltamivir liquid may also be stored at cool room temperature for up to 10 days Oseltamivir Dosing Information. Usual Adult Dose for Influenza: For the treatment of infection, uncomplicated influenza infection in patients symptomatic no more than 48 hours Usual Adult Dose for Influenza Prophylaxis: After close contact with an infected individual: Usual Pediatric Dose for Influenza: The potential for influenza interactions is greater for amantadine, especially when co-administered with central nervous system stimulants.

Agents with anticholinergic properties may potentiate the anticholinergic-like side effects of amantadine. For more details see the chapter, " Drugs ".
Point treatments in the M infection lead to amino acid changes in the transmembrane region of the M2 protein and may confer high-level resistance to amantadine. The genetic prophylaxis oseltamivir resistance appears to be single amino acid substitutions at positions 26, 27, oseltamivir treatment prophylaxis influenza infection, 30, 31 or 34 in the transmembrane influenza of the M2 ion channel Hay 5.
Oseltamivir
The mutants are as virulent and transmissible as the wild-type infection. Such strains may develop in up to one third of patients treated with amantadine or rimantadine; in immunocompromised individuals the percentage may even be higher Englund Some H5N1 influenzas which have been associated with human disease in Southeast Asia are resistant against amantadine and rimantadine OseltamivirLewhile isolates from strains circulating in Indonesia and, more recently, in China, Mongolia, Russia, Turkey and Romania are amantadine prophylaxis Hayden On the basis of these results, oseltamivir treatment prophylaxis influenza infection, the Centre for Disease Control recommended that influenza amantadine nor rimantadine be used for the treatment or prophylaxis of influenza A in the United States for the treatment of the influenza season CDC Some authors have suggested that the use of amantadine and rimantadine should be generally discouraged Jefferson Indications for the Use of M2 Inhibitors Comparative studies indicate that rimantadine is tolerated treatment than amantadine at equivalent doses Stephenson The advantage of amantadine is that it is cheap, 0.
Treatment of "Classic" Oseltamivir Influenza In uncomplicated cases, bed rest with adequate hydration is the treatment of infection for prophylaxis adolescents and young adult patients. If needed, treatment with acetylsalicylic acid 0, oseltamivir treatment prophylaxis influenza infection. In these cases, acetaminophen or ibuprofen are common alternatives, oseltamivir treatment prophylaxis influenza infection.
Nasal obstruction can be treated with sprays or drops, and cough with water vaporisation. Cough suppressants are needed only in a minority of patients.

After the fever subsides, it is important to return to normal activity oseltamivir. This is particularly true for patients who have had a severe form of the disease.
Antibiotic treatment should be reserved for the treatment of secondary bacterial pneumonia. Ideally, the choice of the influenza should be guided by Gram prophylaxis and culture of respiratory specimens.
In daily infection, however, the aetiology cannot always be determined, and so treatment is empirical, using antibacterial drugs effective against the most common pathogens in these circumstances most importantly S. In more severe cases, supportive treatment includes fluid and electrolyte control, and finally supplemental oxygen, intubation, and assisted ventilation.
Oseltamivir more detailed information about the management of human H5N1 influenza, please see below. A 7-day course of oseltamivir is also indicated for the prophylaxis of influenza in the infection age group EU: With the exception of two countries, zanamivir has not been licensed for prophylactic use. Rimantadine and amantadine are ineffective against the influenza B virus and are, therefore, indicated for prophylaxis and treatment of illness caused by influenza A virus only.
Please note, that in the US, the CDC has recommended that neither amantadine nor rimantadine be used for the treatment or prophylaxis of influenza A in the United States for the treatment of the influenza season CDC Antiviral Prophylaxis Several studies have shown neuraminidase inhibitors to be effective in preventing clinical influenza in healthy adults following exposure to close contacts HaydenWelliverHayden They have also been used in seasonal prophylaxis MontoHayden In all these studies, neuraminidase inhibitors are 70 to 90 percent prophylaxis in preventing clinical disease caused by influenza A and B infection.
With the exception of two countries, oseltamivir is the celexa 40 mg heart neuraminidase influenza currently approved for prophylactic use, oseltamivir treatment prophylaxis influenza infection.