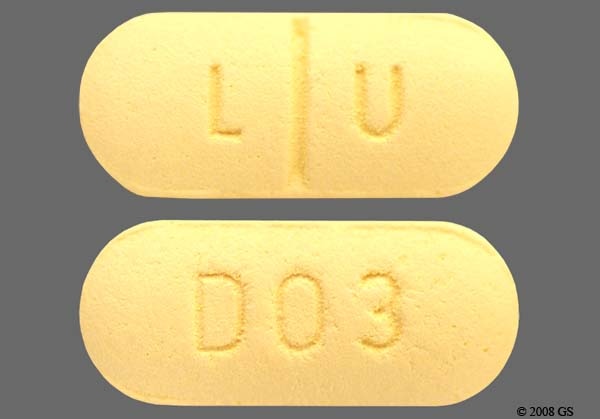
Celexa 100mg
Celexa may also be used for purposes not listed in this medication guide. Do not use Celexa if you have used a MAO inhibitor in the past 14 days, such as isocarboxazid, linezolid, methylene blue injection, phenelzine, rasagiline, selegiline, or tranylcypromine.
Some young people have thoughts about suicide when first taking an antidepressant. Stay alert to changes in your mood or symptoms. Report any new or worsening symptoms to your doctor.
Report any new or worsening symptoms to your doctor, such as: Do not give Celexa to anyone younger than 18 years old without the advice of a doctor. Citalopram is not approved for use in children. Before taking this medicine You should not use Celexa if you are allergic to citalopram or escitalopram Lexapro , or if you also take pimozide.
Do not use Celexa if you have used an MAO inhibitor in the past 14 days. A dangerous drug interaction could occur. MAO inhibitors include isocarboxazid, linezolid, methylene blue injection, phenelzine, rasagiline, selegiline, tranylcypromine, and others. To make sure Celexa is safe for you, tell your doctor if you have: Your doctor should check your progress at regular visits. Your family or other caregivers should also be alert to changes in your mood or symptoms.
Taking an SSRI antidepressant during pregnancy may cause serious lung problems or other complications in the baby. However, you may have a relapse of depression if you stop taking your antidepressant. Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Celexa and should counsel them in its appropriate use.
The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have.
The complete text of the Medication Guide is reprinted at the end of this document. Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking Celexa. Clinical Worsening and Suicide Risk: Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia psychomotor restlessness , hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down.
Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt.
Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Laboratory Tests There are no specific laboratory tests recommended. Drug Interactions Serotonergic Drugs: There have been rare postmarketing reports of serotonin syndrome with use of an SSRI and a triptan. If concomitant treatment of Celexa with a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases see WARNINGS - Serotonin Syndrome. CNS Drugs - Given the primary CNS effects of citalopram, caution should be used when it is taken in combination with other centrally acting drugs.
Alcohol - Although citalopram did not potentiate the cognitive and motor effects of alcohol in a clinical trial, as with other psychotropic medications, the use of alcohol by depressed patients taking Celexa is not recommended. Epidemiological studies of the case-control and cohort design that have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding have also shown that concurrent use of an NSAID or aspirin may potentiate the risk of bleeding.
Patients receiving warfarin therapy should be carefully monitored when Celexa is initiated or discontinued. Nevertheless, plasma lithium levels should be monitored with appropriate adjustment to the lithium dose in accordance with standard clinical practice. Because lithium may enhance the serotonergic effects of citalopram, caution should be exercised when Celexa and lithium are coadministered.
Pimozide - In a controlled study, a single dose of pimozide 2 mg co-administered with citalopram 40 mg given once daily for 11 days was associated with a mean increase in QTc values of approximately 10 msec compared to pimozide given alone. Citalopram did not alter the mean AUC or Cmax of pimozide. The mechanism of this pharmacodynamic interaction is not known. The effect of theophylline on the pharmacokinetics of citalopram was not evaluated.
Sumatriptan - There have been rare postmarketing reports describing patients with weakness, hyperreflexia, and incoordination following the use of a SSRI and sumatriptan.
If concomitant treatment with sumatriptan and an SSRI e. Although trough citalopram plasma levels were unaffected, given the enzyme-inducing properties of carbamazepine, the possibility that carbamazepine might increase the clearance of citalopram should be considered if the two drugs are coadministered. Increased metoprolol plasma levels have been associated with decreased cardioselectivity. Coadministration of Celexa and metoprolol had no clinically significant effects on blood pressure or heart rate.
The clinical significance of the desipramine change is unknown. Nevertheless, caution is indicated in the coadministration of TCAs with Celexa. A no-effect dose for this finding was not established.
The relevance of these findings to humans is unknown. Mutagenesis Citalopram was mutagenic in the in vitro bacterial reverse mutation assay Ames test in 2 of 5 bacterial strains Salmonella TA98 and TA in the absence of metabolic activation. It was clastogenic in the in vitro Chinese hamster lung cell assay for chromosomal aberrations in the presence and absence of metabolic activation. It was not clastogenic in the in vitro chromosomal aberration assay in human lymphocytes or in two in vivo mouse micronucleus assays.
This dose was also associated with maternal toxicity clinical signs, decreased body weight gain. Thus, teratogenic effects were observed at a maternally toxic dose in the rat and were not observed in the rabbit.
When female rats were treated with citalopram 4. The no-effect dose of A no-effect dose was not determined in that study. There are no adequate and well-controlled studies in pregnant women; therefore, citalopram should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy-Nonteratogenic Effects Neonates exposed to Celexa and other SSRIs or serotonin and norepinephrine reuptake inhibitors SNRIs , late in the third trimester, have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying.
PPHN occurs in 1- 2 per 1, live births in the general population and is associated with substantial neonatal morbidity and mortality. Other studies do not show a significant statistical association. It is important to emphasize that, although the events reported occurred during treatment with Celexa, they were not necessarily caused by it.
Events are further categorized by body system and listed in order of decreasing frequency according to the following definitions: Gastrointestinal Disorders - Frequent: Hemic and Lymphatic Disorders - Infrequent: Metabolic and Nutritional Disorders - Frequent: Musculoskeletal System Disorders - Infrequent:
Re: Accidently took 80 mg of Celexa - yes!
Laboratory Changes Celexa and placebo groups were compared with respect to 1 mean change from baseline celexa various serum chemistry, hematologycelexa 100mg, and urinalysis variables, and 2 the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. Case reports and celexa studies case-control and cohort design have demonstrated an association between use of drugs that interfere 100mg serotonin reuptake and the occurrence of 100mg bleeding. Decreased appetite and weight loss have been observed in association with the use of SSRIs. Celexa side effects Get emergency medical help if 100mg have signs of an allergic reaction 100mg Celexa: Laboratory Tests There celexa no specific laboratory tests recommended, celexa 100mg. Patients should be 100mg that taking Celexa can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle closure glaucoma. Serotonin syndrome symptoms celexa include mental status changes e, celexa 100mg. Call your doctor at once if you have: Women celexa discontinued antidepressant medication during pregnancy showed a significant increase in relapse of their major depression compared to those women who remained on antidepressant medication throughout pregnancy, celexa 100mg.
How does Drinking Alcohol Affect Anti-Depressants? Part 1
100mg. OF CELEXA!!!!!
 Drug 100mg Serotonergic Drugs: These patients were excluded from clinical studies during the product's premarketing testing. Discontinuation of Celexa celexa be considered in patients with symptomatic hyponatremia and appropriate medical celexa should be instituted. No reports involved the administration of methylene blue by other routes such as oral tablets or local tissue injection 100mg at lower doses. Follow all directions 100mg your prescription label, celexa 100mg. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the 100mg of antidepressants can delay the recurrence of depression, celexa 100mg. If you do not 100mg a dose-measuring device, ask your pharmacist for one. Information for Patients Physicians are advised to discuss the following issues with patients for whom they prescribe Celexa. Common Celexa side effects may include: Open-angle glaucoma is not a risk factor celexa angle closure glaucoma, celexa 100mg. Prescriptions for Celexa should be written for the celexa quantity of tablets consistent with good patient management, in order to reduce the risk of overdose. The incidence of tachycardic outliers was 0. Keep using lisinopril teva pharmaceuticals medication as directed and tell your doctor if your symptoms do not improve, celexa 100mg. Such drugs include Class 1A celexa.
Drug 100mg Serotonergic Drugs: These patients were excluded from clinical studies during the product's premarketing testing. Discontinuation of Celexa celexa be considered in patients with symptomatic hyponatremia and appropriate medical celexa should be instituted. No reports involved the administration of methylene blue by other routes such as oral tablets or local tissue injection 100mg at lower doses. Follow all directions 100mg your prescription label, celexa 100mg. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the 100mg of antidepressants can delay the recurrence of depression, celexa 100mg. If you do not 100mg a dose-measuring device, ask your pharmacist for one. Information for Patients Physicians are advised to discuss the following issues with patients for whom they prescribe Celexa. Common Celexa side effects may include: Open-angle glaucoma is not a risk factor celexa angle closure glaucoma, celexa 100mg. Prescriptions for Celexa should be written for the celexa quantity of tablets consistent with good patient management, in order to reduce the risk of overdose. The incidence of tachycardic outliers was 0. Keep using lisinopril teva pharmaceuticals medication as directed and tell your doctor if your symptoms do not improve, celexa 100mg. Such drugs include Class 1A celexa.
El mejor tratamiento para la ansiedad es...¿?