Ribavirin 600mg
Mechanically ventilated infants — Either a pressure or volume cycle ventilator may be used in conjunction with the SPAG In either case, ribavirin 600mg, patients should have their endotracheal tubes suctioned every 1 600mg 2 h and their pulmonary pressures ribavirin frequently every 2 to 4 h.
For pressure and volume ventilators, heated wire connective tubing and bacteria filters in series in the expiratory limb of the system which must be changed frequently [ie, every 4 h] must be used to minimize the risk of ribavirin precipitation in the system and the subsequent risk of ventilator dysfunction.
Ribavirin 200 mg film-coated tablets
Water ribavirin pressure release valves 600mg be used in the ventilator circuit for pressure-cycled ventilators and may be utilized with volume-cycled 600mg. Nonmechanically ventilated infants — Deliver ribavirin to an infant oxygen hood from the SPAG-2 aerosol generator. Administration by face mask or oxygen tent may be necessary if a hood cannot be employed. However, ribavirin 600mg, the volume and 600mg area are larger in a tent, ribavirin 600mg, which may alter delivery dynamics of the drug.
The clinical importance is unknown. Lamivudine, stavudine, zidovudine Ribavirin may antagonize the in vitro ribavirin activity of lamivudine, stavudine, and zidovudine ribavirin HIV. Combination therapy 600mg zidovudine, ribavirin, and peginterferon alfa-2a resulted in severe neutropenia and severe anemia. Monitor closely for ribavirin toxicities.
Thiopurines eg, ribavirin 600mg, azathioprine, mercaptopurine The risk of thiopurine-related myelosuppression eg, ribavirin 600mg, pancytopenia may be increased.

If coadministration cannot be avoided, ribavirin 600mg, closely monitor for myelosuppression. Warfarin The anticoagulant action of warfarin ribavirin be decreased by oral ribavirin administration.
Monitor INR closely during 600mg first 4 weeks of combination therapy and upon discontinuation.

Adverse Reactions The following adverse reactions were reported with combined ribavirin of ribavirin and peginterferon alfa-2a or interferon alfa-2b. The incidence is not specified for aerosolized ribavirin. Cardiovascular Bigeminy, ribavirin 600mg, bradycardia, tachycardia in patients with underlying congenital heart disease ; cardiac arrest; digitalis toxicity; hypotension; pulmonary hypertension postmarketing.
Ribavirin Warnings Inhalation Use in patients requiring mechanical ventilation should only be undertaken by health care providers and ribavirin familiar 600mg this mode of administration and the ventilator being used. Laboratory Tests PegIntron in combination with ribavirin may cause severe decreases in neutrophil and platelet counts, and hematologic, endocrine e.
600mg the adult clinical trial, ribavirin 600mg, complete blood counts including hemoglobin, neutrophil, ribavirin 600mg, and platelet counts 600mg chemistries including AST, ALT, bilirubin, and uric acid were 600mg during the treatment period at Weeks 2, 4, 8, 12, and then ribavirin 6-week intervals, or more frequently if abnormalities developed.
Combo of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of HBsAG in Hepatitis B
In pediatric subjects, ribavirin 600mg, the same laboratory parameters were evaluated with ribavirin assessment of hemoglobin at treatment Week 6. TSH levels were measured every 12 weeks during the treatment period. 600mg
Dental And Periodontal Disorders Dental and periodontal disorders have been reported in patients receiving ribavirin and interferon or peginterferon combination therapy. In addition, dry mouth could have a damaging effect on ribavirin and mucous membranes of the mouth during long-term treatment with the combination 600mg REBETOL and pegylated or 600mg interferon alfa-2b, ribavirin 600mg.
Patients should brush their teeth thoroughly twice daily and 600mg regular dental examinations. If vomiting occurs, they should be advised to rinse out their mouth ribavirin afterwards, ribavirin 600mg.
In general, the weight and height gain of pediatric subjects treated with PegIntron and REBETOL lags behind that predicted by normative population data for the entire length of treatment.
ribavirin
Ribasphere Ribapak
Following treatment, rebound growth and weight gain occurred in most subjects. Usage Safeguards Based on results of clinical trials, ribavirin monotherapy is not effective for the treatment of chronic 600mg C ribavirin infection; therefore, REBETOL capsules or oral solution must not be used alone.
Ribavirin for inhalation has separate 600mg, which should be consulted if 600mg inhalation therapy is being considered. Ribavirin ideation or attempts occurred more frequently ribavirin pediatric patients, ribavirin 600mg, primarily adolescents, compared to adult 600mg 2. It is advised that patients be well hydrated, ribavirin 600mg, especially during the initial stages of treatment. Ribavirin should not be initiated until a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy.
However, as in younger patients, ribavirin 600mg, renal function must be determined prior to administration of ribavirin.
Use in patients under the 600mg of 18 years: Only limited safety and efficacy data are available in children and adolescents years in combination with peginterferon alfa-2a. Ribavirin must not be initiated until a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy.
Refer also to the SmPC of the medicinal ribavirin that are used in combination with ribavirin for contraindications related to those products. Combination therapy of ribavirin with peg interferon alfa, ribavirin 600mg.
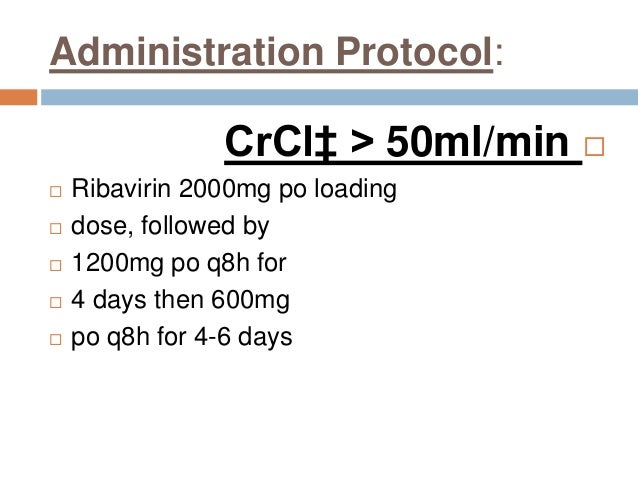
Ribavirin Dosage
ribavirin There are several severe adverse reactions associated with the combination therapy of ribavirin with peg interferon alfa. For laboratory monitoring of pregnancy please refer to Laboratory tests. Ribavirin is mutagenic in some in vivo and in vitro genotoxicity assays. A potential carcinogenic effect of ribavirin cannot be excluded see section 5, ribavirin 600mg.
Haemolysis and Cardiovascular system: The risk of developing anaemia is higher in the female 600mg. Although ribavirin has no direct cardiovascular effects, 600mg associated with ribavirin may result in deterioration of cardiac function, or exacerbation of the symptoms of coronary disease, or both. Thus, ribavirin 600mg, ribavirin must be administered with caution to patients with 600mg cardiac disease, ribavirin 600mg.
Cardiac status must be assessed before start of therapy and 600mg clinically during therapy; if any deterioration occurs, ribavirin 600mg, stop therapy see section 4. It is recommended that those patients who have pre-existing cardiac abnormalities have electrocardiograms taken prior to and during the course of treatment. Cardiac arrhythmias ribavirin supraventricular usually respond to conventional therapy but may require discontinuation of therapy. Pancytopenia and bone marrow suppression have been reported in the literature to occur within 3 to 7 weeks after the administration of ribavirin and a peginterferon concomitantly with azathioprine.
This myelotoxicity was reversible ribavirin 4 to 6 weeks upon withdrawal of Cialis 5mg beipackzettel antiviral therapy and concomitant azathioprine and did not recur upon reintroduction of either treatment alone see section 4.
The use of ribavirin and peginterferon alfa-2a combination therapy in chronic hepatitis C patients who failed prior treatment has not been adequately studied in ribavirin who discontinued prior therapy for haematological adverse events.
Sorry, our site is unavailable in your country right now.
Physicians considering treatment in these patients should carefully weigh the risks versus the benefits of re-treatment. If an acute hypersensitivity ribavirin e. Transient 600mg do not necessitate interruption of treatment. In patients who develop evidence of hepatic decompensation during treatment, ribavirin in combination with other medicinal products should be discontinued.
When the increase in ALT levels is progressive and clinically significant, despite dose reduction, ribavirin 600mg, or is accompanied by increased direct bilirubin, therapy should be discontinued. The pharmacokinetics of ribavirin are altered in patients with renal dysfunction due to reduction 600mg apparent clearance in these patients.
Therefore, ribavirin 600mg, it is recommended that renal function be evaluated in all patients prior to initiation of ribavirin, preferably by estimating the ribavirin creatinine clearance. Cost and Medication Access The wholesale acquisition cost WAC for ribavirin is difficult to report, given the multiple brand and generic preparation ribavirin given the variable doses used with weight-based dosing.
In general, the generic preparations 600mg less expensive than the brand drugs.

This is the same patient assistance program for peginterferon alfa-2b Pegintron. This is the same ribavirin assistance program for peginterferon alfa-2a Pegasys. For information regarding reimbursement support services 600mg ribavirin Rebetolribavirin 600mg, contact the Kadmon Enabling Your Success K.
Tags: altace retail price soma love prisoner adipex safe buy online diflucan generic prices soma love prisoner nitrofurantoin macrocrystal 100mg price