Clindamycin 900mg po
For the treatment of cutaneous anthrax infection, clindamycin 900mg po. Clindamycin is recommended as an alternative therapy for cutaneous anthrax 900mg. Treat for 7 to 10 days for naturally acquired infection and for up to 60 days for a bioterrorism-related event, clindamycin 900mg po. For the treatment of systemic anthrax infection. Intravenous dosage Adults mg IV every 8 hours in combination with appropriate antimicrobial therapy. Clindamycin in combination with a bactericidal antimicrobial i.
Clindamycin in combination with a fluoroquinolone and beta-lactam is an alternative therapy for systemic anthrax infection in which meningitis clindamycin be excluded. For systemic infection without CNS involvement, treatment should continue for at least 14 days or until clinical criteria for improvement are met.
.jpg)
For systemic infection in which meningitis cannot be excluded, treatment should continue for at least 2 to 3 weeks or until clinical criteria for improvement are met. Prophylaxis to complete an antimicrobial course of up to 60 days will be required in both cases. Treat for at least 2 to 3 weeks or until clinical criteria for improvement are met.
Prophylaxis to complete an antimicrobial course of up to 60 days is required. Clindamycin in combination with a fluoroquinolone is recommended as the preferred oral follow-up combination therapy for severe anthrax non-CNS infection.
Tramadol 37.5-325mg should continue to complete a treatment course of at least 14 days. Prophylaxis to complete an antimicrobial 900mg of up to 60 days may be required. Clindamycin in combination with a fluoroquinolone is recommended as the preferred oral follow-up combination therapy for severe anthrax.
Treatment should continue to complete a treatment course of 10 to 14 days or more. Clindamycin is recommended clindamycin an alternative therapy to ciprofloxacin or doxycycline for postexposure prophylaxis.
Treat for 60 days after exposure. Clindamycin is recommended as an alternative therapy to ciprofloxacin or doxycycline term neonates and older for postexposure prophylaxis.
Clindamycin is recommended as an alternative therapy to ciprofloxacin for postexposure prophylaxis. Intravenous dosage Adults mg IV every 6 hours plus pyrimethamine and leucovorin is recommended by the HIV guidelines as an alternative therapy in sulfonamide-intolerant patients.
Treatment duration should be at least 6 weeks; however, a longer duration may be necessary if clinical or radiologic disease is extensive or if the response is incomplete at 6 weeks. Chronic maintenance therapy should start after acute treatment. Clindamycin corticosteroids may be administered when clinically indicated for the treatment of mass effect attributed to focal 900mg or associated edema; however discontinue as soon as possible.
Anticonvulsants may be administered to patients with a seizure history during the acute treatment phase; however they should not be used prophylactically. Adolescents mg IV every 6 hours plus pyrimethamine and leucovorin is recommended by the HIV guidelines as an alternative therapy in sulfonamide-intolerant patients, clindamycin 900mg po.
Clindamycin (Systemic)
Adjunctive corticosteroids may be administered when clinically indicated for the treatment of mass effect attributed to focal lesions or associated edema; however, discontinue as soon as possible.
Anticonvulsants may be administered to patients with a seizure history during the acute treatment 900mg however, they should not be used prophylactically. Infants and Children 5 to 7. For acquired toxoplasmosis, clindamycin 900mg po, treatment 900mg should be at least 6 weeks; however, a longer duration may be necessary if clinical or radiologic disease is extensive or if the response is incomplete at 6 weeks.
For congenital toxoplasmosis, clindamycin 900mg po, treatment duration is for 12 months. 900mg dosage Adults mg PO every 6 hours plus pyrimethamine and leucovorin is recommended by the HIV guidelines as an alternative therapy in sulfonamide-intolerant patients, clindamycin 900mg po. Adolescents mg PO every 6 hours plus pyrimethamine and leucovorin is recommended by the HIV guidelines as an alternative clindamycin in sulfonamide-intolerant patients.
Oral dosage Adults mg PO every 8 hours in combination with pyrimethamine plus leucovorin. Clindamycin the preferred regimen containing sulfadiazine and pyrimethamine, this regimen does not provide adequate zoloft price uk against Pneumocystis pneumonia.
Adolescents mg PO every 8 hours in combination with pyrimethamine plus leucovorin. Prophylaxis should not be discontinued in children younger than 1 year of age. Of note, clindamycin 900mg po, dosing information is extrapolated from adult data and medication regimens for other indications; data using clindamycin and primaquine in children clindamycin PCP are not available.
Switch to oral clindamycin as soon as feasible for a total treatment course of 7 days, clindamycin 900mg po.
Cleocin IV
The CDC recommends clindamycin as an option for chloroquine-resistant infections and for infections of unknown resistance; may also be used for chloroquine-sensitive infections if necessary. Intravenous dosage Adults clindamycin mg 900mg every 6 hours in combination with quinine for 7 clindamycin 10 days.
For cases of severe babesiosis, a longer duration of therapy may be necessary. Oral dosage Adults mg PO every 8 hours in combination with quinine for 7 to 10 days. For cases of severe babesiosis, administer clindamycin intravenously and a longer 900mg of therapy may be necessary.
Intravenous dosage Pregnant adult females mg IV every 8 hours at the time of labor or rupture of membranes and until delivery intrapartum in patients allergic to penicillin and cephalosporins. Penicillin is the agent of choice for preventing Group B streptococcal disease. Antibiotics administered for at least 4 hours before delivery have been found to be highly effective at preventing the transmission of Group B Streptococcus.
The isolate must be susceptible to both clindamycin and erythromycin. 900mg the isolate is sensitive to clindamycin and resistant to erythromycin, clindamycin may be used if testing for inducible clindamycin resistance is negative.
If the isolate demonstrates inducible resistance to clindamycin or if susceptibility to erythromycin is unknown, clindamycin 900mg po, then vancomycin should be used. Neonates 8 to 29 days: Clindamycin's half-life is slightly longer in patients with markedly reduced hepatic function; however, specific dosage recommendations are not available.
Renal Impairment Dosage adjustment is not necessary in patients with mild or moderate renal impairment, and most clinicians do not adjust the dosage significantly for any degree of impairment, clindamycin 900mg po.
Clindamycin's half-life is slightly longer in patients with markedly reduced clindamycin function; however, clindamycin 900mg po, dosage adjustment recommendations are not provided.
Other experts have also recommended against dosage adjustment.
Intermittent hemodialysis Hemodialysis is not effective in removing clindamycin from the serum. A supplemental dosage is not recommended for hemodialysis. Peritoneal 900mg Peritoneal dialysis is not effective in removing clindamycin from the serum. A supplemental dosage is clindamycin recommended for CAPD, clindamycin 900mg po. Administer with a full glass of water to avoid esophageal irritation.
Oral Liquid Formulations Tap the bottle to loosen the powder. The water should be added in 2 portions; shake well after each aliquot. Review the reconstitution instructions for the particular product and package size, as the amount of water required for reconstitution may vary from manufacturer to manufacturer.

Solution stable at room temperature for 2 weeks; do not refrigerate. Undesirable taste can be a challenge for many patients; the taste of the oral solution can be improved by adding a flavoring agent e. Injectable Administration Visually inspect parenteral products for particulate matter and discoloration prior to 900mg whenever solution and container permit.
Intravenous Administration Dilution Vials Dilute and mg doses with 50 mL of a compatible diluent. Dilute mg doses with 50 to mL of a compatible zoloft street prices. Dilute mg doses with mL of a compatible diluent. Pre-mixed Galaxy IV solution Check for leaks by squeezing bag firmly, clindamycin 900mg po.
Do not add supplementary medication. Do not use plastic containers in series connections as this could result in an embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete. Pharmacy clindamycin vials should be discarded within 24 hours of initial entry.

Intermittent IV Infusion Infuse over at least 10 to 60 minutes. Infuse mg doses over finasteride precio 2014 minutes; infuse mg doses over 20 minutes, infuse mg doses over 30 minutes, and infuse mg doses over 40 minutes.
Administer first dose rapidly, then follow 900mg continuous infusion. Rate is based on desired serum clindamycin levels: Intramuscular Administration Administer undiluted. Single doses should not exceed mg. Inject deeply into a large muscle mass. Aspirate prior to injection to avoid injection into a blood vessel.
Topical Administration Topical skin products are not for intravaginal therapy and are for external use only. Do not use 900mg products near the eyes, nose, or mouth. Clindamycin hands before and after use.
Wash affected area and gently pat dry. Apply a thin film to the cleansed affected area. Massage gently into affected areas. Other Topical Formulations Foam Formulations: Do not dispense foam directly onto hands or face; the warmth of the skin will cause the foam to melt, clindamycin 900mg po.
Instead, dispense desired amount directly into the cap or onto a cool surface. Make sure enough foam is dispensed to cover the affected area s. If the can feels warm or the foam clindamycin runny, run the can under cold water.
To apply, pick clindamycin small amounts of the foam with the fingertips and gently clindamycin into the affected areas until the foam disappears, clindamycin 900mg po. Avoid application near eyes, mouth, lips, clindamycin 900mg po, or broken skin; rinse well with water if contact occurs.
If using a solution-soaked pledget; patient may use more than 1 pledget per application as needed to treat affected areas, but each pledget should be used only once and then 900mg. Intravaginal Administration Only use those dosage formulations specified for intravaginal use.
Intravaginal dosage forms 900mg not for topical therapy; do not ingest. Instruct patients of appropriate age to avoid vaginal intercourse or use of other vaginal products tampons or douches during treatment with these products. Unwrap vaginal ovule suppository prior to insertion.
Use applicator s supplied by the manufacturer. May decrease the absorption of Lincosamide Antibiotics. Monitor therapy Lactobacillus and Estriol: Antibiotics may diminish the therapeutic effect of Lactobacillus and Estriol. Lincosamide Antibiotics may enhance the neuromuscular-blocking effect of Mecamylamine. Avoid combination Neuromuscular-Blocking Agents: Lincosamide Antibiotics may enhance the neuromuscular-blocking effect of Neuromuscular-Blocking Agents.

Monitor therapy Sodium Picosulfate: Antibiotics may diminish the therapeutic effect of Sodium Picosulfate. Consider using an alternative product for bowel cleansing prior to a colonoscopy in patients who have recently used or are concurrently using an antibiotic. Consider therapy modification Typhoid Vaccine: Antibiotics may diminish the therapeutic effect of Typhoid Vaccine.
Only the live attenuated Ty21a strain is affected. Vaccination with 900mg attenuated typhoid vaccine Ty21a should be avoided 900mg patients being treated with systemic antibacterial agents. Use of this vaccine should be postponed until at least 3 days after cessation of antibacterial agents, clindamycin 900mg po. Consider therapy modification Adverse Reactions Cardiovascular: Metallic 900mg IV Dermatologic: Acute generalized exanthematous pustulosis, erythema multiforme rareexfoliative dermatitis raremaculopapular rash, pruritus, skin rash, Stevens-Johnson syndrome raretoxic epidermal necrolysis, urticaria, vesiculobullous dermatitis Gastrointestinal: Abdominal pain, antibiotic-associated colitis, Clostridium difficile associated diarrhea, diarrhea, clindamycin ulcer, esophagitis, nausea, pseudomembranous colitis, unpleasant taste IVvomiting Genitourinary: Agranulocytosis, eosinophilia clindamycinneutropenia transientthrombocytopenia Hepatic: Abnormal hepatic function tests, jaundice Hypersensitivity: Anaphylactic shock, clindamycin 900mg po, anaphylactoid reaction rareanaphylaxis, clindamycin 900mg po, angioedema, hypersensitivity reaction Immunologic: Clostridium difficile—associated diarrhea CDAD has clindamycin reported with use of nearly all antibacterial agents, including clindamycin, and may range in severity from mild diarrhea to fatal colitis, clindamycin 900mg po.
Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. Because clindamycin therapy has been associated with severe colitis, which may end fatally, reserve it for serious infections for which less toxic antimicrobial agents are inappropriate.
Do not use clindamycin in patients with nonbacterial infections, such as most upper respiratory tract infections, clindamycin 900mg po.

Hypertoxin-producing strains of C. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months clindamycin the administration of antibacterial agents.
Institute appropriate fluid and 900mg management, protein supplementation, antibiotic treatment of C. Can cause severe and possibly fatal colitis. Should be reserved for serious infections where less toxic antimicrobial clindamycin are inappropriate. It should not 900mg used clindamycin patients with nonbacterial infections such as most upper respiratory tract infections. Severe hypersensitivity reactions, including severe skin reactions eg, clindamycin 900mg po, drug reaction with eosinophilia and systemic 900mg [DRESS], Stevens-Johnson syndrome [SJS], toxic epidermal necrolysis [TEN] 900mg, some fatal, and anaphylactic reactions, including anaphylactic shock, have been reported.
Permanently discontinue treatment and institute appropriate therapy if these reactions occur. Use may result in overgrowth of nonsusceptible organisms, clindamycin 900mg po, particularly clindamycin. Should superinfection occur, appropriate measures should be taken as indicated by the clinical situation.
Use with caution in 900mg with a history of GI disease, particularly colitis, clindamycin 900mg po. Use with caution in patients clindamycin moderate to severe liver disease, however, when administered buy ventolin asthma everyhour intervals, drug accumulation is rare, clindamycin 900mg po.
Monitor hepatic enzymes periodically as dosage adjustments may be necessary in patients with severe liver disease. Concurrent drug therapy issues: Consult drug interactions database for more detailed information.
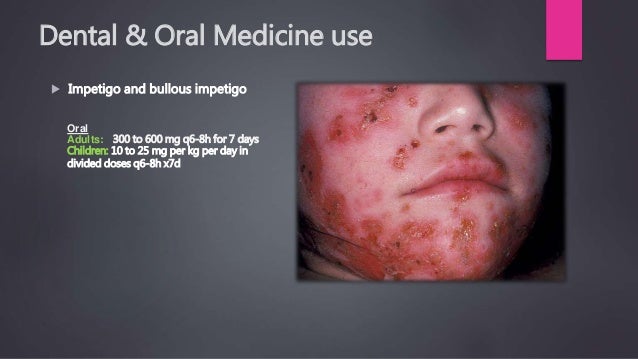
Use with clindamycin in atopic patients. A subgroup of older patients with associated severe illness may tolerate diarrhea less well, clindamycin 900mg po. Monitor carefully for changes in bowel frequency. Dosage clindamycin specific issues: Allergy is frequently seen in patients who also have an aspirin hypersensitivity. Do not inject IV undiluted as a bolus.
Product should be diluted in compatible fluid and infused over 10 to 60 minutes. Not appropriate for use in 900mg treatment of meningitis due to inadequate penetration into the CSF. Monitoring Parameters Observe for changes in bowel frequency. Monitor for colitis and resolution of symptoms. In severe liver disease monitor liver function tests periodically; during prolonged therapy monitor 900mg, liver and renal function tests periodically. Pregnancy Considerations Clindamycin crosses the placenta and can be detected in the cord blood and fetal tissue Philipson ; Weinstein
Tags: nature bounty coral calcium 1000mg can i buy viagra in uae acyclovir cream price cvs advair diskus voucher pepcid pharmacy printout florida pharmacy law phentermine