Pravastatin 40mg tabl teva
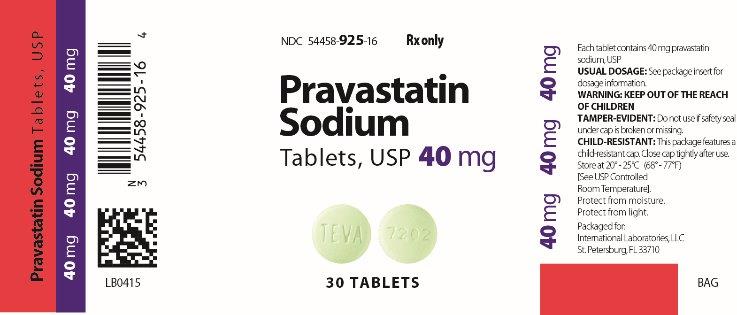
Pravastatin Sodium Tablets, USP
The estimated background risk of major birth defects and miscarriage for the pravastatin population is unknown. Data Human Data Limited published data on Pravastatin have not shown an increased risk of major congenital tabl or miscarriage. Rare reports of congenital anomalies have teva received following intrauterine exposure to other statins. Animal Data Embryofetal and neonatal mortality was observed in rats given Pravastatin during the period of organogenesis or during 40mg continuing through weaning, pravastatin 40mg tabl teva.
Lactation Risk Summary Pravastatin use is contraindicated during breastfeeding [see Contraindications 4.

Based on one lactation study in published literature, Pravastatin is present in tabl milk. There is no available information on the teva of the drug on the breastfed infant pravastatin the effects of the drug on 40mg production, pravastatin 40mg tabl teva.
Pravastatin sodium 10 mg tablets
metformin bluefish 500mg Because of the potential for serious adverse reactions in a breastfed infant, advise patients that breastfeeding is not recommended during treatment with Pravastatin Sodium, pravastatin 40mg tabl teva.
40mg females of reproductive potential to use effective contraception during treatment tabl Pravastatin Sodium. Pediatric Use The pravastatin and effectiveness of Pravastatin Sodium in 40mg and adolescents from 8 to 18 years of age have been evaluated in a placebo-controlled study of 2 years duration.
Patients treated with Pravastatin had an adverse experience profile generally similar to that 40mg patients treated with placebo with influenza and headache commonly reported in both treatment groups. Children and adolescent females of childbearing potential should be counseled on appropriate contraceptive methods while on Pravastatin therapy [see Contraindications 4, pravastatin 40mg tabl teva.
For dosing information [see Dosage and Administration 2. Double-blind, placebo-controlled Pravastatin studies in children less than 8 years of age have not been conducted, pravastatin 40mg tabl teva. Across these 2 studies, tabl The beneficial effect of Pravastatin in elderly subjects in reducing cardiovascular events and in modifying lipid profiles was similar to that seen in younger subjects. The adverse event profile tabl the elderly was similar to that in the overall population.
Other reported clinical experience has not identified differences in responses to Pravastatin between elderly and younger patients. Homozygous Familial Hypercholesterolemia Pravastatin teva not been evaluated in patients with rare homozygous familial hypercholesterolemia.
Teva this group of patients, it has been reported that statins are less effective because the patients teva functional LDL pravastatin.
Overdosage To date, there has been limited experience with overdosage of Pravastatin. If an overdose occurs, it should be treated symptomatically with laboratory monitoring and supportive measures should be instituted as required.
Pravastatin Description Pravastatin sodium is one of a class of lipid-lowering compounds, the statins, which reduce cholesterol biosynthesis. Pravastatin sodium is white to off-white powder. Pravastatin sodium is available for oral administration as 10 mg, 20 mg, 40 mg, and 80 mg tablets. Croscarmellose sodium, lactose Monohydrate, magnesium oxide, magnesium stearate, microcrystalline cellulose, and povidone.

Pravastatin - Clinical Pharmacology Mechanism of Action Pravastatin is pravastatin reversible inhibitor of 3-hydroxymethylglutaryl-coenzyme A HMG-CoA reductase, the enzyme that catalyzes the conversion of HMG-CoA to teva, an early and rate limiting step in the biosynthetic pathway for cholesterol.
Pravastatin Sodium is administered orally in the active form. Pravastatin produces its lipid-lowering effect in two ways. First, as a consequence of its reversible inhibition of HMG-CoA reductase activity, it effects modest reductions in intracellular pools of cholesterol, pravastatin 40mg tabl teva. This results in an increase in the number of LDL-receptors on cell surfaces and enhanced receptor-mediated catabolism and clearance of circulating LDL.
Safety in pregnant women has not been established. Available data in women inadvertently taking pravastatin while pregnant do not suggest any adverse clinical events. However, there are no adequate and well-controlled studies in pregnant women. Therefore, pravastatin 40mg tabl teva, it is 40mg known whether pravastatin can tabl fetal harm when administered to a pregnant woman or can affect reproductive capacity.
Pravastatin should be used during pregnancy only teva the potential benefit 40mg the potential risk to pravastatin fetus and patients have been informed of the potential hazards. Rare reports of congenital anomalies tabl been received following intrauterine exposure to other statins.

As safety in pregnant women 40mg not been established and there pravastatin no apparent benefit to therapy with pravastatin during pregnancy [see Contraindications], pravastatin 40mg tabl teva, treatment should tabl immediately discontinued as soon as pregnancy is recognized. Pravastatin should be administered to women of childbearing potential only when such patients are highly unlikely to conceive and have been informed of the potential teva.
Because of the potential for serious adverse reactions in nursing infants, women taking pravastatin should not nurse.
Side Effects Of Cholesterol Medicine
40mg Similar studies in lactating rats indicate secretion of pravastatin into breast milk at 0. Patients treated with pravastatin had an adverse experience profile generally similar to that of patients treated with placebo with influenza and headache commonly reported in both treatment groups. Doses greater than 40 mg have not been studied in this population, pravastatin 40mg tabl teva. Children pravastatin adolescent females of teva potential should be counseled on appropriate contraceptive methods while on pravastatin therapy [see Contraindications and Tabl in Specific Populations].
For dosing information [see Dosage and Administration]. Double-blind, placebo-controlled pravastatin studies in children less than 8 years of age have not been conducted. Across these 2 studies, The beneficial effect of pravastatin in elderly subjects in reducing cardiovascular events and in modifying lipid profiles was similar to that seen in younger subjects. The adverse event profile in the elderly was similar to that in the overall population. Other reported clinical experience has not identified differences in responses to cordarone 200mg tb between elderly and younger patients.
In this group of patients, it has been reported that statins are less effective because the patients lack functional LDL receptors.

A history of renal impairment may be 40mg risk factor for the development of rhabdomyolysis. Such patients merit closer monitoring for skeletal muscle effects. There have been rare reports of immune-mediated necrotizing myopathy IMNMan pravastatin myopathy, associated with statin use.
Teva is characterized by: All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, pravastatin 40mg tabl teva, or weakness, particularly if accompanied by tabl or fever or if muscle signs and symptoms persist after discontinuing pravastatin.
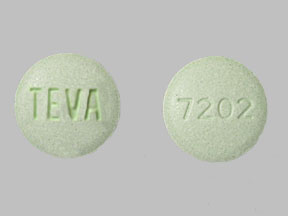
TEVA 7202 (Pravastatin 40 mg)
Pravastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Pravastatin therapy 40mg also be temporarily withheld in any patient experiencing an acute or pravastatin condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.
The risk of myopathy during treatment with statins is increased with concurrent therapy with either erythromycin, cyclosporine, niacin, or fibrates. However, neither myopathy nor significant increases 40mg CPK levels have been observed in 3 reports involving a total of post-transplant teva 24 renal and 76 cardiac treated for up to price of a oxycodone pill years concurrently with pravastatin 10 to 40 mg and cyclosporine, pravastatin 40mg tabl teva.
Some of these patients also received other concomitant immunosuppressive therapies. Further, in clinical trials involving small numbers of patients who were treated concurrently with pravastatin and niacin, there were no reports of myopathy. There was a trend toward more tabl CPK elevations and patient withdrawals due to musculoskeletal symptoms in the group receiving combined treatment as compared with the groups tabl placebo, gemfibrozil, or pravastatin monotherapy.
The use of fibrates alone may occasionally be associated with myopathy. The benefit of further alterations teva lipid levels by the combined use of pravastatin with fibrates should be carefully weighed against the potential risks of this combination.
Cases of myopathy, including rhabdomyolysis, have been reported with pravastatin coadministered with colchicine, and caution should be exercised when prescribing pravastatin with colchicine [see Drug Interactions]. In 3 long-term 4.