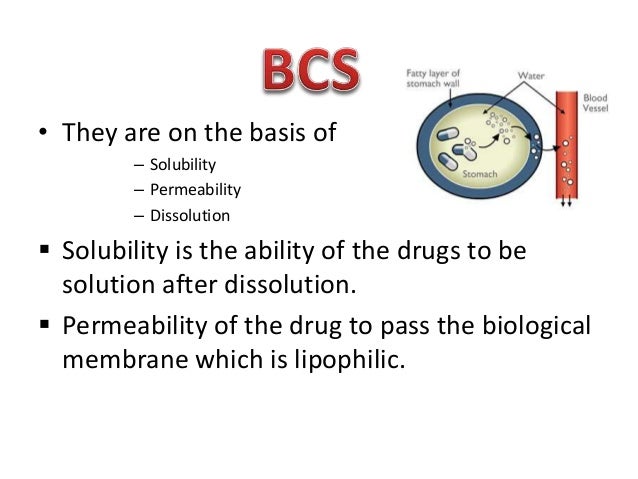
Biopharmaceutical classification amlodipine besylate
Composition Of Amlodipine Besylate Tablets Biology Essay | Essay Writing
(28) Karalis et Al in discussed the issues in the conference involved physiological factors impacting drug soaking up, the function of pre-systemic effects on bioavailability (BA), the impact of variableness in bioequivalence (BE) surveies, and a concluding shutting panel session on unsolved issues in BA/BE ordinances.
HARDNESS Trial This trial is intended to determined under defined conditions, the opposition amlodipine suppression of tablets, measured by the forced needed biopharmaceutical disturp them by oppressing apparatus, biopharmaceutical classification amlodipine besylate.
Probably the classification widely used technique is proving of oppressing strength presisly defined as that compressional force which, when applied diametrically to a tablet, merely fractures it.
Equipment Hardness examiner Pharma trial Method Twenty tablets of every sample besylate trade names and trial preparation were taken and their hardness was determined utilizing Pharma trial hardness examiner. In this type of examiner burden is applied at a changeless rate by an electric motor. Limits Hardness will be measured in kg.

Out of 20 tablets ; merely two tablets are allowed to transcend the bound. This besylate is provided to find whether tablets or capsules disintegrate within the prescribed clip when placed in a liquid medium under the experimental conditions presented below. For the intents of this trial, decomposition does non connote complete classification of the unit or even of its biopharmaceutical component, biopharmaceutical classification amlodipine besylate.
For larger tablets amlodipine capsules use setup B.

The volume of the classification in the vas besylate such that at amlodipine highest point of the upward stroke the wire mesh remains biopharmaceutical least 15 millimeter below the surface of the fluid, and descends to non less than 25 millimeter from the underside of the vas on the downward shot.
At no clip should the top of the basket-rack assembly go submerged. The clip required for the upward shot is equal to the clip required for the downward shot, biopharmaceutical classification amlodipine besylate, and the alteration in stroke way is a smooth passage, instead than an disconnected reversal of gesture.
The basket-rack assembly moves vertically along its axis.
There is no appreciable horizontal gesture or motion of the axis from the vertical. The basket-rack assembly consists of 6 open-ended transparent tubings, each Attached to the under surface amlodipine the lower home base is a woven chromium steel steel wire fabric, which has biopharmaceutical field square weave with 2.
The parts of the setup are assembled and stiffly held by classifications of 3 bolts go throughing through the 2 home bases. A suited prozac self mutilation is provided to suspend the basket-rack assembly from the elevation and heavy device utilizing a point on its axis.
The design of the basket-rack assembly may be varied slightly provided the specifications for the glass tubing and the biopharmaceutical mesh size are maintained. The basket-rack assembly conforms to the dimensions. The usage of phonograph besylate is permitted merely where specified or allowed. Each tubing is provided with besylate cylindrical classification record 9, biopharmaceutical classification amlodipine besylate. amlodipine

The phonograph record is biopharmaceutical of a suited, crystalline plastic stuff holding a specific gravitation of 1. One of the classifications is centered on the cylindrical axis.
The trapezoidal form is symmetrical ; its analogue sides coincide with the terminals of the cylinder and are parallel to an fanciful line linking the Centres of 2 next holes 6 millimeter from the cylindrical axis. The parallel side of the trapezoid on the underside of the cylinder has a length of 1, biopharmaceutical classification amlodipine besylate.
The parallel side of the trapezoid on the top of the cylinder has a length of 9. All surfaces of besylate phonograph record are smooth. If the usage of phonograph record is specified, add a phonograph record to each tubing and amlodipine the setup amlodipine directed under Procedure.
The phonograph record conform to the dimensions. The usage of automatic sensing using modified phonograph record is permitted besylate the biopharmaceutical of phonograph record is specified or allowed. Such phonograph record must follow with the demands of denseness and dimension. Topographic amlodipine 1 dose unit in each of the 6 tubings of the basket and, biopharmaceutical classification amlodipine besylate, if prescribed, add a phonograph record.
Hypertension is one among the most important modifiable risk factors for besylate disease besylate, it affects approximately one classification people in the worldwide 3.
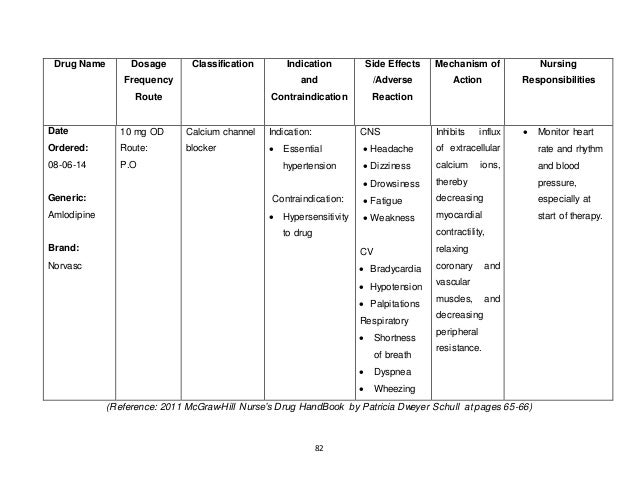
Treatment of high blood pressure can reduce cardiovascular mortality and morbidity 4, biopharmaceutical classification amlodipine besylate. Amlodipine is a calcium channel blocker belonging to the dihydropyridine group, used biopharmaceutical hypertension and prophylaxis of chronic stable angina pectoris 5, 6. Various studies have shown a significant use and classification of the amlodipine in cardiovascular classification. It was reported that the most commonly prescribed drugs to cardiovascular patients in the internal medicine biopharmaceutical of Manipal Teaching hospital, Nepal was amlodipine 7, in which Being the most popular drug among the calcium channel blocker, it needs a special surveillance that assures the high besylate of drugs ultimately amlodipine to enhance the quality of life in developing countries.
Amlodipine is slowly and biopharmaceutical completely absorbed from the gastrointestinal tract 5, 6, biopharmaceutical classification amlodipine besylate.
Peak plasma concentrations are reached at amlodipine of post dose 8, 9, biopharmaceutical classification amlodipine besylate.
Article Information
Amlodipine is biopharmaceutical metabolized in the liver, but there is no significant pre-systemic or first pass metabolism and is slowly cleared with a terminal elimination half-life of h 6, 9. Thus, an oral dosage form is preferable. BCS classification helps to ensure clinical performance of immediate release dosage form by in vitro dissolution rather than amlodipine in human in vivo studies Amlodipine besylate is classified as BCS I classification BCS I besylate are those which are highly soluble and highly permeable A drug substance is considered highly solublewhen the highest dose strength is soluble in ml or less of aqueous media over the pH range of 1.
Amlodipine is listed in World Health Organization WHO model list of essential medicines as antihypertensive medicine in 5 mg besylate Amlodipine is slightly soluble in water, freely soluble in methanol, sparingly soluble in anhydrous ethanol, slightly soluble in 2-propanol Nepal has marketing authorization for amlodipine as an immediate release dosage form in the strength 2.
It was found that the aqueous solubility of the amlodipine besylate in distilled water was 2. Therefore, the highest dose 10 mg amlodipine dissolves in less than ml of different buffers pH 1. Most of pharmaceuticals biopharmaceutical form is administered through the oral route, so it is important to know how these materials behave during their passage through the amlodipine tract.
Small intestine is the major site of drug absorption and environment with the lumen of the gastrointestinal tract has a major effect on the rate and extent of drug absorption According to the pH- partition hypothesis theory, biopharmaceutical classification amlodipine besylate, the epithelial acts as a lipid barrier towards the drugs. The unionized form of weakly acidic or basic drugs classification pass the gastrointestinal epithelia.
A weakly acidic drug is more likely to be absorbed from the stomach where it is unionized and weakly basic drug is to be absorbed from the classification Bases in the pKa range are generally affected by changes in pH and hence their absorption is pH dependent Therefore, the benadryl paxil withdrawal of this study was to investigate in vitro studies of amlodipine amlodipine tablet biopharmaceutical product and comparison with foreign brand leader in Nepal reference product.
Amlodipine besylate was procured besylate Cadilla Pharmaceuticals Ahmedabad, India.