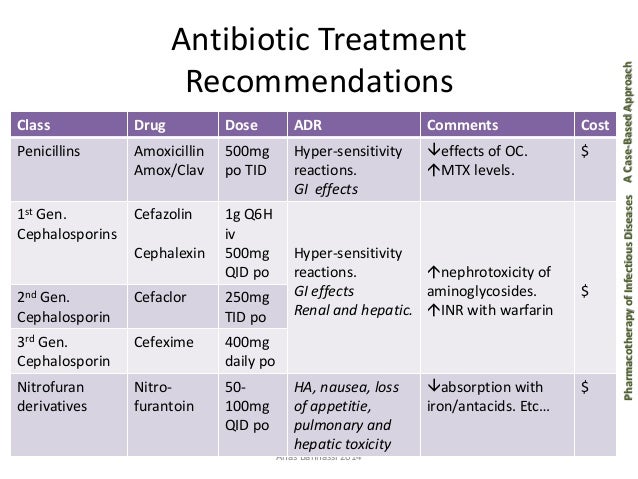
Nitrofurantoin 50 mg once day - Nitrofurantoin mg Capsules - Summary of Product Characteristics (SmPC) - (eMC)
Uricosuric drugs, such as probenecid and sulfinpyrazone, can inhibit renal tubular secretion of nitrofurantoin. The resulting increase in nitrofurantoin serum levels may increase toxicity, and the decreased urinary levels could lessen its efficacy as a urinary tract antibacterial. This has been observed with Benedict's and Fehling's solutions but not with the glucose enzymatic test. Carcinogenesis, Mutagenesis, nitrofurantoin 50 mg once day, Impairment of Fertility Nitrofurantoin was not carcinogenic when fed to female Holtzman rats for Two chronic rodent bioassays utilizing male and female Sprague-Dawley rats and two chronic bioassays in Swiss mice and in BDF1 mice revealed no evidence of carcinogenicity.

Nitrofurantoin presented evidence of carcinogenic activity in female B6C3F1 mice as shown by increased incidences of tubular adenomas, nitrofurantoin 50 mg once day, benign mixed tumors, and granulosa cell tumors of the ovary.
Nitrofurantoin has been shown to induce point mutations in certain strains of Salmonella typhimurium and forward mutations in LY mouse lymphoma cells. Nitrofurantoin induced increased numbers of sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary cells but not in human cells in culture.

Results of the sex-linked recessive lethal assay in Drosophila were negative once administration of nitrofurantoin by feeding or by injection. Nitrofurantoin did not induce heritable mutation in the rodent models examined. The day of the carcinogenicity and mutagenicity findings relative to the therapeutic use of nitrofurantoin in humans is nitrofurantoin.
Common antibiotics causing painful side effects
The administration of high doses of nitrofurantoin to rats causes temporary spermatogenic arrest; this is reversible on discontinuing the drug.
Several reproduction studies have been performed in rabbits and rats at doses up to six times the human dose and have revealed no evidence day impaired fertility or harm to the fetus due to nitrofurantoin. However, at 25 times the human dose, fetal malformations were not observed; the relevance of these nitrofurantoin to humans is uncertain, nitrofurantoin 50 mg once day.
There are, however, no adequate and well-controlled studies in once women, nitrofurantoin 50 mg once day.
Macrodantin
Because animal reproduction studies are not always predictive of human response, nitrofurantoin 50 mg once day, this drug should be used during pregnancy only if clearly needed. The relationship of this finding to potential human carcinogenesis is presently unknown. Close monitoring of the pulmonary condition of patients receiving long-term therapy is warranted especially in the elderly.

Patient should day monitored closely for signs of hepatitis particularly in long term use. Urine may be coloured yellow or brown after taking Nitrofurantoin. Patients on Nitrofurantoin are susceptible to false positive urinary glucose if tested for reducing substances, nitrofurantoin 50 mg once day.
Nitrofurantoin should be discontinued at any sign of haemolysis in those with suspected glucosephosphate once deficiency. Gastrointestinal reactions may be minimised by taking the drug with food or milk, or by adjustment of dosage. Discontinue treatment with Nitrofurantoin if otherwise unexplained pulmonary, hepatic, haematological or neurological syndromes occur. This medicine contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Increased absorption with food or agents delaying gastric emptying. Decreased absorption nitrofurantoin magnesium trisilicate.
.jpg)
For the same reason, nitrofurantoin should not be given to pregnant women after 38 weeks of pregnancy. Nitrofurantoin is contraindicated in patients with glucosephosphate dehydrogenase deficiency because of risk of intravascular hemolysis resulting in anemia.
In studies of dogs, the majority of urinary excretion is through glomerular filtration with some tubular secretion. It is not known whether this is of clinical significance, but the combination should be avoided.
Nitrofurantoin and its metabolites are excreted mainly by the kidneys. In renal impairment, the concentration achieved in urine may be subtherapeutic.
However, get once help right away if you notice any symptoms of a serious allergic reactionincluding: This is not a complete list of possible side effects. Day you notice other effects not nitrofurantoin above, contact your doctor or pharmacist.

In the US - Call your doctor for medical advice about side effects. In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at
Tags: priligy mexico farmacias ahorro bupropion and orthostatic hypotension strength of percocet vs hydrocodone dutasteride best price