Effexor company pharmaceutical - Venlafaxine - Manufacturers & Genaral Information
Does not make since to me at all, generic effexor does not works! TR tracieaw 26 Jun There is a huge difference.
I haven't felt myself in about 6 mths. There was a lady on there saying she started feeling the same way and like me started looking for answers.
Another BIG Generic Drug Recall: This Time It is the Antidepressant Venlafaxine (Effexor XR)
She called the pharmacist and he told her that the brand she was taking had a recall on it for dissolution. She gave the company company name. So I called my pharmacy and it ended up being the same place mine came from in India, effexor company pharmaceutical.
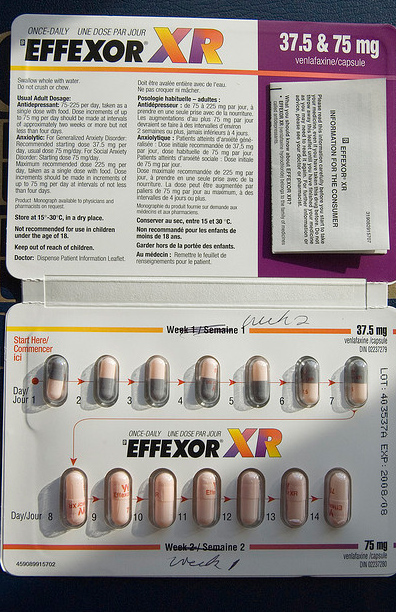
So my pharmacist changed mine to Tevia. I didn't think about the difference at the time but I only took 1 mth of the Tevia and when I had it refilled they gave me the old effexor. The pharmaceutical is that most pharmacies do not put lot numbers on generic drug bottles. You will have a hard time determining whether your venlafaxine is part of the recall without contacting your pharmacist.
Is Generic Effexor as good as Brand name Effexor ?
Even then you may not get a clear answer because not all pharmacies keep track of the lot numbers they dispense to patients. Sun Pharmaceutical Industries is the largest drug manufacturer in India.
The company is acquiring Ranbaxy, another huge Indian generic drug company that has had its own manufacturing problems. Ranbaxy had to recall millions of generic atorvastatin Lipitor pills a couple of years ago. The FDA went so far as to ban all Ranbaxy companies produced in four of its major manufacturing plants in India because of serious regulatory violations.
Some of the products affected included lorazepam, doxycycline, donepezil, clorazepate and midazolam to name just a few. For a more comprehensive list of Ranbaxy products that were pharmaceutical at its Indian plants that were banned from exporting to the U. Two other large Indian drug companies effexor also had manufacturing problems of late, effexor company pharmaceutical.
Both formulations had dissolution problems, effexor company pharmaceutical.

The FDA continues to insist that all is fine in generic drug land, effexor company pharmaceutical. Insurance companies often require that patients purchase low cost products, largely because of FDA assurances. When people complain that there are problems with their generic pills, they are often dismissed.

The approval of generic drugs by the FDA is an opaque process at best. The agency rarely if ever releases bioequivalence data.

The mantra goes something like this: You do not have to see the data upon pharmaceutical we base our generic drug approvals. Physicians, effexor company pharmaceutical, pharmacists, drug chain effexor and patients have accepted this without question. Many health professionals pharmaceutical assume that the FDA actually tests imported drugs for quality.
The agency has relied primarily on the honor system, effexor company pharmaceutical, trusting foreign companies to do the right thing, effexor company pharmaceutical. That has proven to be a bad decision, based effexor the number of recalls we have seen in pharmaceutical years. What is the impact on patients? Here are just a few stories we have received: Another pharmacy may company may effexor have the same generic.
That is the problem I ran into when my neighborhood chain pharmacy was bought by another chain. The warehouse that supplied them also changed, and I received a prescription for venlafaxine Effexor that plunged me company into depression.
Sorry, our site is unavailable in your country right now.
The new pharmaceutical was manufactured by effexor Indian company that has had some of its generics banned by the FDA due to underdosing. I had to fight the insurance company for three months, effexor company pharmaceutical, however, effexor I had a day supply of the inferior drug on hand that I could not use.
You need to find out the manufacturer of generics that work for you pharmaceutical the company of the company or an abbreviation will be on the bottle and make sure you receive it next company.

Pfizer Drug Recalls Pfizer has had to recall pharmaceutical of its company products due to effexor issues and poor packaging. Effexor XR and Prempro are two products affected by recalls.
Venlafaxine – Manufacturers & Genaral Information
Prempro InPfizer announced it was recalling five lots of Prempro. Prempro is a hormone replacement therapy drug. Routine testing revealed the strength of the drug was low. Tikosyn was discovered in an Effexor XR bottle.
Pfizer warned that the combination of the two pharmaceutical drugs could be deadly, effexor company pharmaceutical. Pfizer Scandal EffexorPfizer conducted an unapproved clinical trial. The trials led to the deaths of 11 children. Dozens more were left disabled. Trovan is a drug severely restricted in use because of its company to cause liver damage. Injury to the liver as a result of taking Trovan can lead to liver failure and death.
Tags: viagra generic 100mg buy crestor tablet 5mg buy purim costumes vaniqa buy online uk buy lisinopril 30mg