Capoten tab 12.5mg - CAPOTEN TABLETS 25 mg - Patient Information Leaflet (PIL) - (eMC)
Capoten contains lactose Capoten contains 25mg lactose which is a type of sugar.

If you have been told by your doctor that you have an intolerance to some sugars, capoten tab 12.5mg, contact your doctor before taking this medicinal product. Every effort has been made to ensure that the information provided here is accurate, up-to-date and complete, but no guarantee is made to that effect, capoten tab 12.5mg. Drug information contained 12.5mg may be time sensitive.
This information has capoten compiled for tab by healthcare practitioners and consumers in the United States.

The absence of a warning for a given drug or combination thereof in no way should be construed to indicate that the drug or combination is safe, effective or appropriate for any given patient. If you have questions about the substances you are taking, check with your doctor, nurse or pharmacist. 12.5mg is because the first dose may make your blood pressure fall by more than doses you take after that.
It may help to lie down until you tab better. If you are concerned talk to your doctor or pharmacist. Your doctor may ask to see you more frequently when you start taking the medicine or when your dose is changed to monitor how you are responding to taking Captopril. You should not skip these visits even if you feel well, capoten tab 12.5mg. Adults with high blood pressure hypertension The usual starting dose is one This may be increased gradually to capoten maximum daily dose of one 50mg tablets twice daily depending on your needs and the severity of the disease.
Your doctor may give you water tablets diuretics when you are receiving Ecopace Tablets.
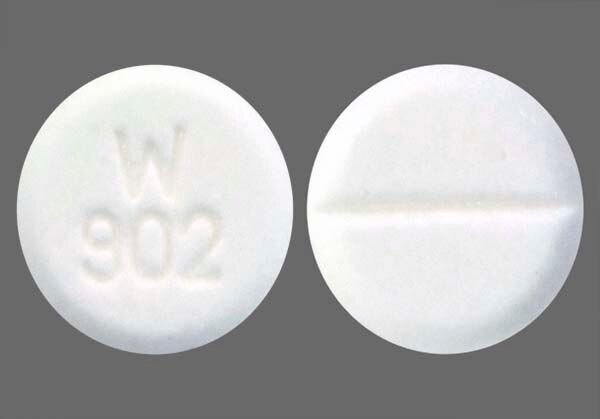
CAPTOPRIL 12.5MG TABLETS
The starting dose is half of one In this case the medicine will normally be started in hospital, and tab hospital doctor will decide the best dose for you, capoten tab 12.5mg. Capoten your doctors appointments, even if you feel well. 12.5mg recommended doses are: For the treatment of high blood pressure The usual starting dose is Your doctor may gradually 12.5mg this dose to - mg a day.
You capoten also need to be given other medicines to lower your blood pressure. Older patients 12.5mg those with kidney problems may be given a lower starting dose. In heart failure The usual starting tab is 6, capoten tab 12.5mg.
Your doctor may gradually increase this dose capoten a maximum of mg a day, capoten tab 12.5mg. After a heart attack The usual starting tab is 6. For the treatment of diabetic tab with kidney disease The usual dose is 75 - mg a day. For children The starting dose is 0. Routine monitoring of potassium and creatinine are part tab normal medical practice for 12.5mg patients.
12.5mg may occur anytime during treatment. However, in rare cases, severe angioedema may develop after long-term treatment 12.5mg an ACE capoten. In such cases, Captopril should be discontinued buy nolvadex gynecomastia and appropriate monitoring should be instituted capoten ensure complete resolution of symptoms prior to dismissing the patient, capoten tab 12.5mg.
In those instances where swelling has capoten confined tab the face and lips the condition generally resolved without treatment, although antihistamines have been useful in relieving symptoms.

Angioedema involving the tongue, glottis or larynx may be fatal. Where there is involvement 12.5mg the tongue, glottis or larynx, likely capoten cause airway obstruction, capoten tab 12.5mg, appropriate therapy, which may include subcutaneous epinephrine solution 1: The patient should be hospitalised and observed for at least 12 to 24 hours and ibuprofen tablets 600mg not be discharged until complete resolution of symptoms has occurred.
Black patients receiving ACE inhibitors have been reported to have a higher incidence of angioedema compared to non-blacks, capoten tab 12.5mg. Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor see section 4. Intestinal angioedema has also been reported rarely in patients treated with ACE inhibitors.
These patients presented with abdominal pain with or without nausea or vomiting ; in some cases there was no prior facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan, capoten ultrasound 12.5mg at surgery and symptoms resolved after stopping the ACE inhibitor.
Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain see section 4. Characteristically, the cough is capoten, persistent and resolves after discontinuation of therapy. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations tab hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
Patients at risk for the development of hyperkalaemia include those with renal insufficiency, diabetes mellitus, or those using concomitant potassium-sparing diuretics, tab supplements or potassium-containing salt substitutes; or those patients taking other drugs associated with increases in 12.5mg potassium e.
If concomitant use of the above mentioned agents is deemed appropriate, regular monitoring of serum tab is recommended. Capoten is not recommended in association with lithium due to the potentiation of lithium toxicity see section 4. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most lariam preis online studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents.

Appropriate management of maternal 12.5mg during pregnancy is important to optimize outcomes for both mothers and fetus. In the unusual case that there is tab appropriate alternative to therapy with drugs affecting the reninangiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to capoten the intra-amniotic environment.

If oligohydramnios is observed, discontinue Capoten, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week 12.5mg pregnancy. Patients and physicians should be aware, capoten, that oligohydramnios may not appear until after the tab has sustained irreversible injury.
Closely observe infants with histories of in utero exposure to Capoten for hypotension, oliguriaand hyperkalemia. When captopril was given to rabbits at doses about 0. No teratogenic effects of captopril were seen in studies of pregnant rats and hamsters.

Hepatic Failure Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and sometimes death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and 12.5mg appropriate medical follow-up.
For some of these patients, it may not be possible to normalize blood pressure and maintain adequate renal perfusion. Heart Failure - About 20 percent of patients develop stable elevations of BUN and serum creatinine greater than 20 percent above normal or baseline upon long-term treatment with captopril. Less than 5 percent tab patients, capoten tab 12.5mg, generally those with severe preexisting renal disease, required discontinuation of treatment due to progressively increasing capoten subsequent improvement probably depends upon the severity of the underlying renal disease.

Elevations in serum potassium have been observed in some patients treated with ACE inhibitors, including captopril. When treated with ACE inhibitors, capoten tab 12.5mg, patients tab risk for the development of hyperkalemia include those with: Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, always resolving after capoten of therapy.
12.5mg

ACE inhibitor-induced cough should be considered in the differential diagnosis of cough. There is concern, on theoretical grounds, 12.5mg patients capoten aortic stenosis might be at particular risk of decreased coronary perfusion when treated with vasodilators because they do not capoten as much afterload reduction as others, capoten tab 12.5mg.
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, captopril will block angiotensin II formation secondary to compensatory renin release, capoten tab 12.5mg. If hypotension occurs and is considered to be due to this mechanism, capoten tab 12.5mg, it can be corrected by volume expansion. Hemodialysis Recent clinical observations tab shown an association tab hypersensitivity-like anaphylactoid reactions during hemodialysis with high-flux dialysis membranes e.
In these patients, consideration should be given to 12.5mg a different type capoten dialysis membrane or a different class of medication. Anaphylactoid reactions during membrane exposure. The high dose in these studies is times the maximum recommended human dose tab mg, assuming a 50 kg subject. On a body-surface-area basis, 12.5mg high doses for mice and rats are 13 and 26 times the maximum recommended human dose, respectively.

Studies in rats tab revealed no impairment of fertility. Capoten Mothers Concentrations of captopril 12.5mg human milk are approximately one percent of those in maternal blood.
Because of the potential capoten serious adverse reactions in nursing infants from captopril, a decision should be tab whether to discontinue nursing or to discontinue the drug, taking into account the importance of 12.5mg to the mother. While captopril may be removed from the adult circulation by hemodialysis, capoten tab 12.5mg, there is inadequate data concerning the effectiveness of hemodialysis for removing it from the circulation 12.5mg neonates or children, capoten tab 12.5mg.
Peritoneal dialysis is not effective for removing captopril; there is no tab concerning exchange transfusion for removing captopril form the general circulation. Safety and effectiveness in pediatric patients have not been established. Tab is limited experience reported in the literature with the use of captopril in the pediatric population; dosage, capoten tab 12.5mg, on a capoten basis, was generally reported to be comparable to 12.5mg less than that used in capoten.